

Type 1 diabetes is an autoimmune disease that causes the body to attack the insulin-producing beta cells of the pancreas.1 There are many different areas of research related to diabetes, and one of them is preventing type 1 diabetes.
There is a large group of healthcare professionals and scientists who are working in collaboration through the group Trial Net (trialnet.org), with the goal of preventing type 1 diabetes. They have been working since 1994 to identify people who have diabetes autoantibodies, and then studying ways to slow or hopefully stop the onset of diabetes.2
Last week, a group of 18 diabetes thought leaders, including various endocrinologists, diabetes healthcare professionals, and CWD’s own Jeff Hitchcock, spoke to the Food & Drug Administration’s (FDA) Endocrinologic and Metabolic Drugs Advisory Committee about a new promising trial drug to help delay the onset of type 1 diabetes.3 The data from Provention Bio’s trial therapy, Teplizumab, was reviewed and determined to be ready for review by the FDA for possible approval.3
Teplizumab has been studied through phase 2 clinical trials, meaning that it was tested in people who were identified to have diabetes auto-antibodies.4 The study results from the phase 2 trial were very promising, with a reduction in risk of diagnosis of diabetes from 35.9% per year in the placebo group to 14.9% per year in the treatment group.4 (What’s a Placebo?)
Dr. Carla Greenbaum participated in our Screenside Chat series this past January 2021, and used this visual to describe how the drug had worked for prevention:
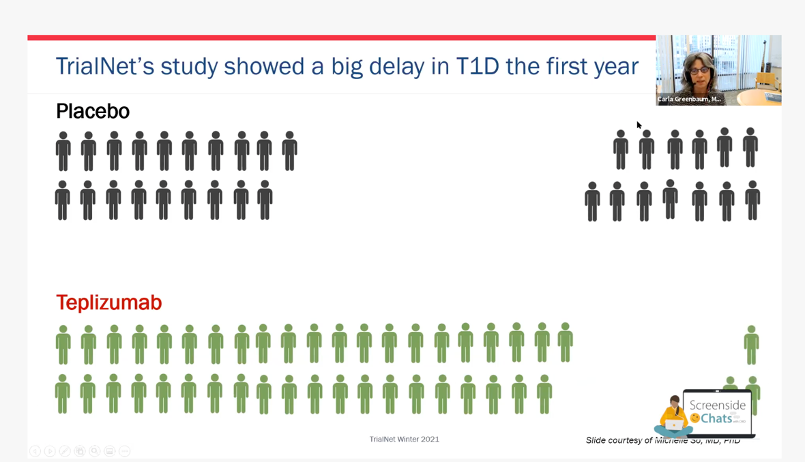

The image shows how many people progressed to having diabetes after the study, and the results were very promising! You can watch the full Screenside Chat with Trialnet here.
Another interesting part about the Teplizumab study from 2019 was that it seemed participants with specific antibodies responded better to the drug than those without those same antibodies.4 This shows further evidence for the need for more research and more options for preventative measures. As Dr. Carla Greenbaum says in the Screenside Chat, “What we really need is more clinical trials, so we have more therapies … one option isn’t good enough … the key here is we want multiple options because we can delay or prevent diabetes in more people with multiple options.”
Although this may not help those of us who live with diabetes, CWD’s Founder & President, Jeff Hitchcock, stated during his testimony to the FDA on May 27, 2021, “If you can lessen the burden, delay the burden, and perhaps even prevent the burden, you must do so.”
Here’s to hope for the future!
- What is Diabetes
- TrialNet: Our Research
- FDA Advisory Committee Votes in Favor of the Benefits of Teplizumab Outweighing the Risks in Support of Approval to Delay Clinical Type 1 Diabetes (T1D)
- An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes

