FDA approval for Control IQ has arrived!
According to the press release from December 13, 2019, “The U.S. Food and Drug Administration today authorized marketing of the Tandem Diabetes Care Control-IQ Technology, an interoperable automated glycemic controller device that automatically adjusts insulin delivery to a person with diabetes by connecting to an alternate controller-enabled insulin pump (ACE pump) and integrated continuous glucose monitor (iCGM).”
This is huge news from the San Diego-based diabetes company, who announced over the summer that it is offering no-cost feature updates for t:slim X2 insulin pump customers in
“Today’s action continues the agency’s ongoing efforts to work with the diabetes community to help ensure the safety and efficacy of innovative and customizable diabetes management systems that may help patients better tailor their treatments to their individual needs,” said Tim Stenzel, M.D., Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, in today’s press release from the FDA. “The marketing authorization of this first stand-alone interoperable automated glycemic controller also allows substantially equivalent controller technologies that are developed for diabetes in the future to go through the 510(k) review process, helping to promote timely patient access to innovative technologies that can improve their care and quality of life.”
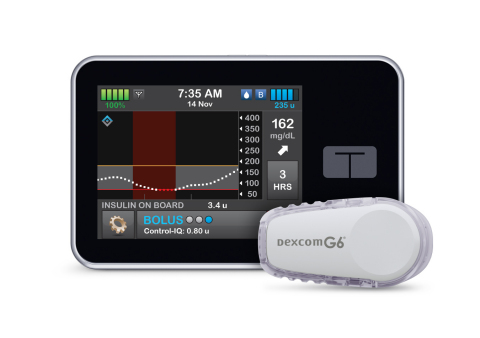
The team at Tandem Diabetes Care put out a press release this morning, as well, and including some benefits of Control-IQ Advanced Hybrid Closed-Loop Technology:
“Predicts and helps prevent lows and highs – Control-IQ technology uses CGM readings to predict glucose values 30 minutes ahead and can increase, decrease or stop basal insulin delivery to help keep glucose in range (70-180 mg/dL) (1).
Automatic Correction Boluses – If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus with a target of 110 mg/dL and delivers 60 percent of that value. It will do this up to once an hour as needed.
Accommodates for sleep and exercise – Control-IQ technology offers optional settings for sleep and exercise that change the treatment values to better match the different physiologic needs during these activities.
No fingersticks – With Dexcom G6 CGM integration, the Control-IQ feature works with no fingersticks required for mealtime dosing or calibration (2). Other benefits of the Dexcom G6 CGM include an extended 10-day wear, acetaminophen blocking (6), and the ability to share real-time CGM data with up to 10 followers (7)
Easy to use – The system has no complicated criteria to keep Control-IQ technology on. If the CGM signal is temporarily lost, the Control-IQ feature will resume automatically when the CGM is back in range. In the pivotal study, participants gave Control-IQ technology a 4.7 out of 5.0 for ease of use, and a 4.8 out of 5.0 for desire to continue use of the system (8).
Standard Features of the t:slim X2 Insulin Pump:
Color touchscreen – The large color touchscreen on the t:slim X2 pump is easy to read, simple to learn, and intuitive to use for anyone familiar with a smartphone or tablet.
Small and discreet – The t:slim X2 pump is up to 38 percent smaller than other pumps (9), yet can hold up to 300-units of insulin.
Can be used with or without the Control-IQ feature or CGM – When advanced features are turned off, the t:slim X2 pump removes the CGM chart from the screen and puts the Bolus and Option buttons front and center for easy access.”
The Children with Diabetes team is very excited about today’s approval of Control IQ. “With today’s announcement of FDA approval of Tandem’s Control-IQ, people with type 1 diabetes have an amazing new tool to help them thrive,” said CWD President, Jeff Hitchcock. “The clinical trial result from Control-IQ, published in The New England Journal of Medicine in October 2019, showed dramatic increases in time in range and dramatic reductions in time spent in hyper- and hypoglycemia. We applaud everyone involved in bringing Control-IQ to patients and look forward to continuing innovation in automated insulin delivery systems.”
“We believe that by continuing to offer new technologies that are easy to use, we will drive greater customer adoption. And it’s working,” said John Sheridan. “We continue to see that approximately half of our new pump users are people who have never used an insulin pump before. This supports our longer term goal to bring the benefits of pump therapy to more people with diabetes, especially those who have been discouraged from using insulin pump technology in the past.”
“Control-IQ clearance isn’t a step for automated insulin delivery, it’s a leap,” said Sheridan.
1 As measured by CGM
2 If glucose alerts and CGM readings do not match symptoms or expectations or if taking over the recommended maximum dosage amount of 1000mg of acetaminophen every 6 hours, use a blood glucose meter to make diabetes treatment decisions.
3 Dexcom G6 CGM sold separately
4 The Dexcom G6 CGM transmitter can only be paired with one medical device (either a
6 Dexcom G6 CGM readings can be used to make diabetes treatment decisions when taking up to a maximum acetaminophen dose of 1,000mg every 6 hours. Taking a higher dose may affect the G6 readings.
7 Separate Follow App required.
8
9 38 percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe and Omnipod System. Data on file,

